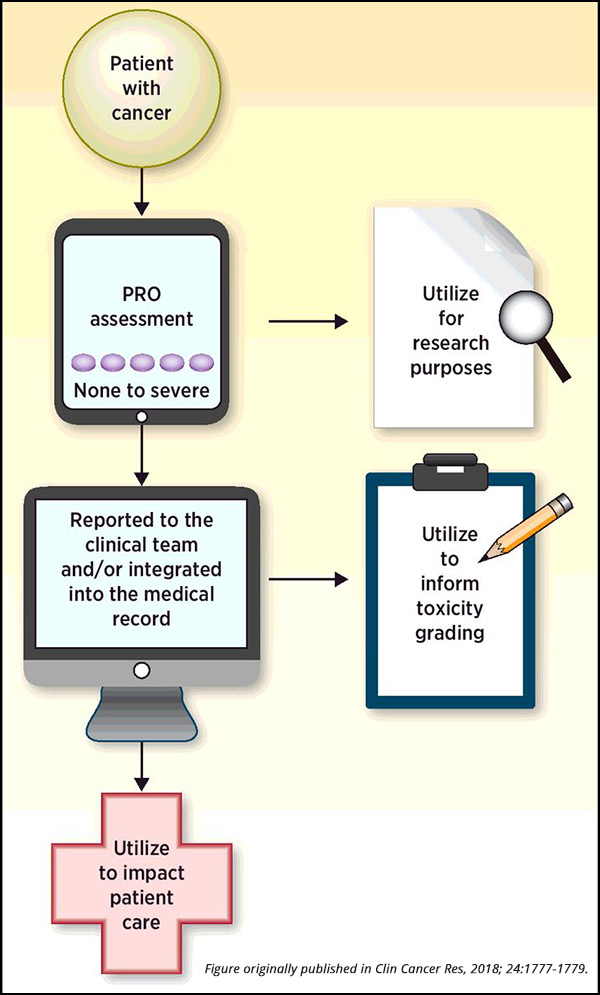
Utilizing Patient-reported Outcomes in Cancer Clinical Trials
Assessing new anticancer therapeutics in clinical trials is a vital step in evaluating the toxicity and efficacy of treatments before their approval for widespread use. Throughout these trials, many patients encounter a variety of side effects, ranging from physical ailments such as nausea or rash to psychological symptoms such as depression or anxiety. While it is standard practice to record clinician-reported outcomes to characterize safety, there is no current standard requirement for the use of patient-reported outcomes (PROs) in cancer clinical trials.
Assessment of safety in cancer clinical trials
All cancer clinical trials require the collection of clinician-assessed adverse events (AEs). AEs can range from laboratory findings to clinical events (such as stroke or blood clot) or physical symptoms. The clinician will assign a grade to each AE in a standard fashion using the Common Terminology Criteria for Adverse Events (CTCAE). Reported AEs from grade 1 (mild) to grade 4 (life-threatening/disabling) or even grade 5 (death) are used to guide treatment dosages and to characterize the toxicity profile of the therapy.
While clinician-reported AEs focus on safety and toxicity, PROs highlight the symptomatic adverse events that can affect the tolerability of the treatment from the patient’s perspective, explained Paul Kluetz, MD, acting associate director, Oncology Center of Excellence at the U.S. Food and Drug Administration (FDA). Kluetz is the senior author of a recent article published in Clinical Cancer Research providing current thinking from several government agencies on the use of PROs to inform tolerability in cancer clinical trials.
The safety profile of a therapy, as reported by a clinician, is typically described in the FDA label and in most publications by the documentation of the highest grade of a particular AE that occurred at any time while the patient was on trial, Kluetz added. The patients, however, can provide additional information about their experience – how severe was the symptom from the patient’s perspective? Did the symptom interfere with the patient’s usual activities? At what point did the symptom occur during the treatment and did it persist or resolve?
“PROs are a type of measurement – a source of data,” said Kluetz. “A PRO result is based on a report that comes directly from the patient, and these assessments can be incorporated into clinical trials to quantify the symptoms and the functional impacts of the disease as well as the therapy under study, providing a more comprehensive assessment of the risks and benefits of a particular treatment.”
Importantly, data garnered from PRO assessments are not meant to be directly compared to clinician-reported outcomes, noted Kluetz. As the information comes from two distinct sources (clinicians or patients), PRO data is meant to complement standard clinician reporting. Any differences in the two sources should not be used to inform deficiencies in the quality of clinician-reported toxicity assessments. “Currently, systematic PRO measurements are generally reviewed at trial completion to better understand and communicate the profile of AEs to clinicians and future patients who will undergo similar treatments,” said Kluetz.
Many different tools exist to document patient-reported symptoms. As described in the companion article in Clinical Cancer Research, common platforms include the Edmonton Symptom Assessment System (ESAS), the Memorial Symptom Assessment Scale (MSAS), the Rotterdam Symptom Checklist, the MD Anderson Symptom Inventory (MDASI), and the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).
PRO measures are routinely captured in commercial clinical trials. While this PRO data has been historically analyzed at the end of the study to describe the patient experience, there is interest in using the PRO results generated from symptom questions to monitor symptoms and improve supportive care, explained Kluetz. Research has suggested that using PROs to assist in the monitoring of symptoms can result in improved clinical outcomes, and even increase patient survival.
While these early results are encouraging, this practice remains uncommon and is not required by the FDA. “Despite early data regarding the potential utility of using PRO measures to communicate patient-reported symptoms in real time to health care providers, the use of PROs to guide care is still not routine in clinical practice, and is rare in cancer clinical trials,” noted Kluetz.
PRO-CTCAE
“A successful PRO strategy starts with sponsors and investigators doing the work to ask the right questions to meet trial objectives, and to use PRO questionnaires that are fit for that purpose,” Kluetz said.
The National Cancer Institute (NCI) developed PRO-CTCAE to complement CTCAE, the standard platform used by clinicians to report adverse events in clinical trials. The PRO-CTCAE library represents 78 symptomatic AEs for patients to communicate longitudinal data on symptoms that can inform the tolerability of their treatment.
“One of the biggest problems that we see with PROs is the use of generic questions that may not be specific to the trial,” explained Kluetz. “This can result in duplicative questions, measurements of symptoms that may not be occurring, and missed assessments of important symptomatic toxicities.
“PRO-CTCAE is unique in that it is a library of many symptoms designed specifically to assess patient-reported symptomatic adverse events to complement standard clinician CTCAE reporting,” said Kluetz. “It is more flexible than the historical use of PRO measures because the investigator can select a tailored subset of questions depending on the expected side effects of the trial. This flexibility is sorely needed given how many different toxicity profiles exist in contemporary drug development.”
Current challenges and next steps for further PRO incorporation
It is common to have PRO measures assessed in randomized trials submitted to the FDA. While PRO-CTCAE data and other data on patient-reported symptoms and function can inform the overall safety and tolerability profile of a therapy upon trial completion, logistical issues will need to be addressed if PRO symptom measures will be incorporated into the clinical monitoring of patients during trial conduct. More will be learned about the use of PROs to improve patient-physician communication in the clinical care context, and this experience can inform future discussions on the strengths and limitations of including real-time clinical monitoring of PRO results in the trial context, noted Kluetz. Importantly, Kluetz states, “Regardless of the monitoring strategy employed in the trial, it is critical that patients be regularly informed regarding how their PRO data will be used and should always be educated to contact their health care team directly for any concerning signs or symptoms.”
Patient willingness to complete PRO assessments does not appear to be a major problem in the implementation of PROs in cancer clinical trials. One study assessed the utilization of PRO-CTCAE in a multicenter clinical trial, and found that 86 percent of patients self-reported their symptoms during active treatment, and 72 percent self-reported symptoms following treatment. Interestingly, two of the common reasons that led to a lack of patient reporting included technical issues, such as internet connectivity and forgetting to provide computers to patients. Other common factors included patients missing clinic appointments and feeling “too sick” to self-report.
“Obtaining rigorous PRO data also means verifying that patients complete their assessments,” noted Kluetz. “This is done by educating both patients and the trial sites about the importance of these questions and instituting monitoring strategies to ensure completion.”
As described in a policy review recently published in Lancet Oncology, there is an increasing global interest in the rigorous implementation of PROs into cancer clinical trials. International collaboration and ongoing improvements in standardization of PRO instruments, assessments, and analytics will hopefully facilitate the incorporation of PRO measures into increasing numbers of cancer clinical trials.
Editor’s note: The opinions offered by Kluetz in this post may not necessarily reflect all authors’ views involved in the aforementioned article. The perspectives discussed here should not be construed to represent official views or policies of the FDA, the NCI, the Office for Human Research Protections, or the Department of Veterans Affairs.